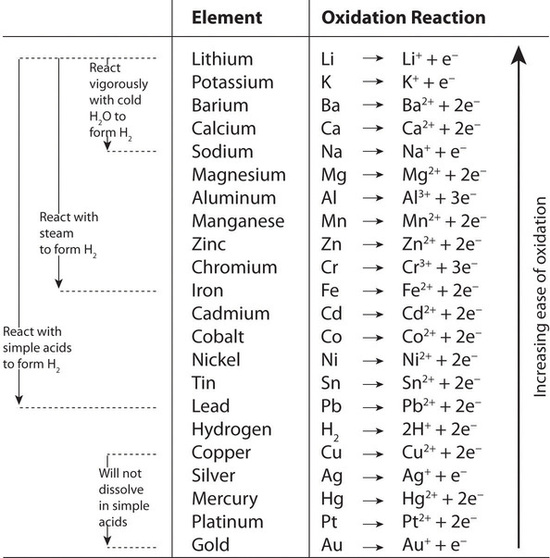
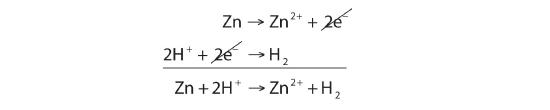SOLVED:Which metal can be oxidized with an \mathrm{Sn}^{2+} solution but not with an \mathrm{Fe}^{2+} solution?

A redox reaction occurs between aluminum and chromium with the half- reactions shown. What can you conclude about the spontaneity of the reaction? Al3+(aq) + 3e− Al(s) Eº = −1.66 V Cr(s) Cr3+(aq) +

A redox reaction occurs between aluminum and chromium with the half- reactions shown. What can you conclude about the spontaneity of the reaction? Al3+(aq) + 3e− Al(s) Eº = −1.66 V Cr(s) Cr3+(aq) +

PDF) Designing a Graphene Coating-Based Supercapacitor with Lithium Ion Electrolyte: An Experimental and Computational Study via Multiscale Modeling

PDF) Designing a Graphene Coating-Based Supercapacitor with Lithium Ion Electrolyte: An Experimental and Computational Study via Multiscale Modeling

A redox reaction occurs between aluminum and chromium with the half- reactions shown. What can you conclude about the spontaneity of the reaction? Al3+(aq) + 3e− Al(s) Eº = −1.66 V Cr(s) Cr3+(aq) +

PDF) Designing a Graphene Coating-Based Supercapacitor with Lithium Ion Electrolyte: An Experimental and Computational Study via Multiscale Modeling

Redox reactions of cobalt, aluminum and titanium substituted lithium manganese spinel compounds in lithium cells - ScienceDirect